Generic Drugs: What They Are, How They Work, and What You Need to Know
When you hear generic drugs, pharmaceutical products that contain the same active ingredients as brand-name drugs but are sold under their chemical name. Also known as generic medications, they are the backbone of affordable healthcare in the U.S. and around the world. Most people assume they’re just cheaper copies—but that’s not the full story. The FDA requires generic drugs to have the same strength, dosage form, route of administration, and therapeutic effect as their brand-name counterparts. That means if your doctor prescribes lisinopril, the generic version of Zestril, it’s not a substitute—it’s the exact same medicine, just without the marketing budget.
But here’s what most patients don’t realize: FDA approval, the rigorous process that ensures generic drugs meet the same safety and effectiveness standards as brand-name drugs isn’t just about the final pill. The FDA inspects every manufacturing facility—no notice, no exceptions. They test raw ingredients, production lines, and even how the drug breaks down in your body. If a generic doesn’t absorb the same way, it gets rejected. That’s why a generic metformin from a reputable pharmacy works just as well as Glucophage. But not all pharmacies source from the same suppliers, and that’s where things get tricky. Some online sellers push counterfeit versions that look real but contain nothing—or worse, dangerous fillers like fentanyl. Always get your generics from a licensed pharmacy, not a website that looks like it was built in 2003.
Then there’s the drug equivalence, the scientific proof that a generic performs the same as the brand-name version in clinical use. It’s not just about the active ingredient. Fillers, binders, and coatings can affect how quickly the drug enters your bloodstream. For most people, that difference is negligible. But if you’re on thyroid meds like levothyroxine, blood thinners like warfarin, or seizure drugs like phenytoin, even tiny changes in absorption can throw off your whole treatment. That’s why some patients stick with the brand—because their body reacts differently to the generic version. It’s not placebo. It’s physiology.
And cost? Generic drugs cut prices by 80% on average. Synthroid might cost $60 a month. Levothyroxine? $10. But here’s the catch: if you switch back and forth between different generic brands, your body might react to each change in filler. That’s why your doctor might write "Dispense as written" on your prescription—to keep you on the same version. Don’t assume all generics are interchangeable. Ask your pharmacist: "Which manufacturer makes this?" and stick with it.
There’s no magic here. Generic drugs aren’t second-rate. They’re the result of science, regulation, and competition. But they’re not foolproof. The system works—when you know how to use it. Below, you’ll find real guides on how to spot safe generics, why some people react differently to them, how the FDA keeps them honest, and what to do when your insurance pushes a brand you can’t afford. These aren’t theoretical articles. They’re what real patients need to know before they pick up their next prescription.
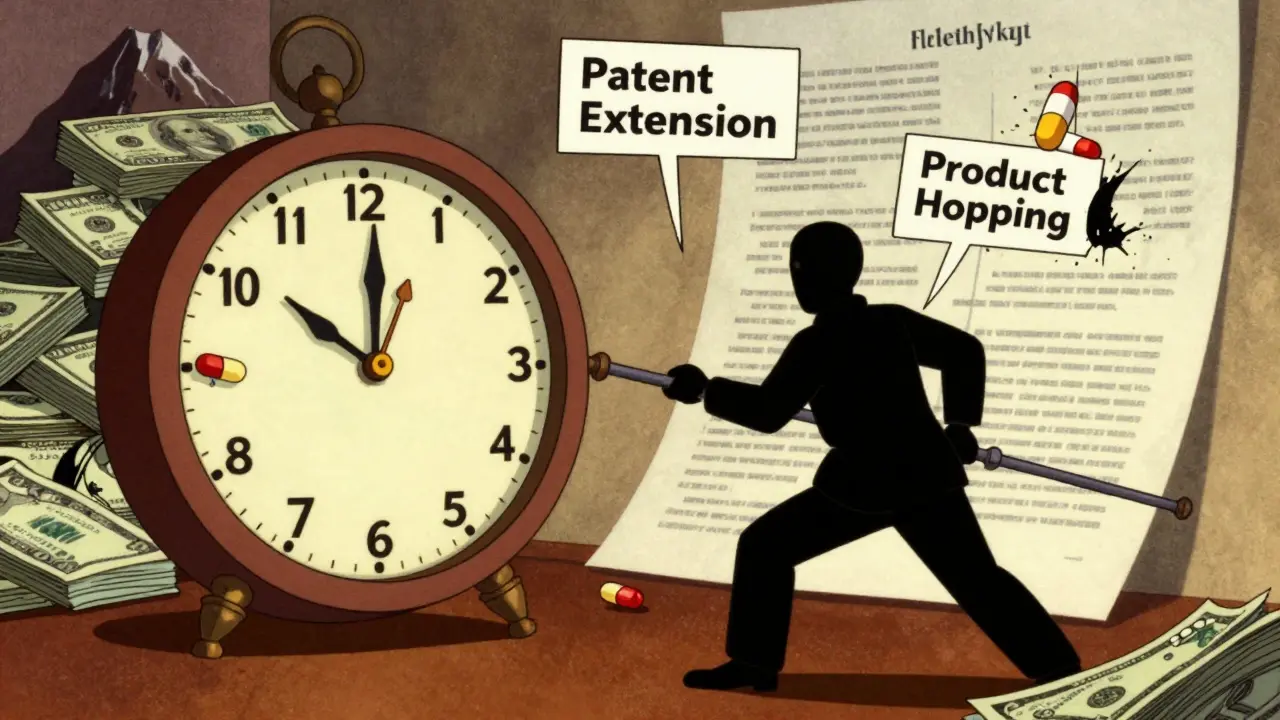
Evergreening: How Pharmaceutical Brands Delay Generic Drugs to Protect Profits
Evergreening lets drug companies extend patent life with minor tweaks, blocking affordable generics and keeping prices high. Learn how it works, why it's legal, and how it hurts patients.
How to Ask Your Doctor About Generic Alternatives: Save Money Without Compromising Care
Learn how to ask your doctor about generic alternatives to save hundreds or thousands on prescription meds without sacrificing effectiveness. Real cost savings, expert-backed advice.
Authorized Generics vs Traditional Generics: What You Need to Know
Learn the key differences between authorized generics and traditional generics, how they're made, why it matters for your health, and when to ask for one over the other. Not all generics are the same.
Why Most Drugs Don't Have Authorized Generics - And What It Means for Your Prescription Costs
Most prescription drugs don't have authorized generics - even expensive ones. Learn why brand companies choose which drugs get cheaper versions, how it affects your costs, and what you can do about it.
Medicare Part D Formularies: How Generic Coverage Works in 2025
Learn how Medicare Part D formularies cover generic drugs in 2025, including tiered pricing, the $2,000 out-of-pocket cap, and how to save hundreds on prescriptions. Updated for current rules.
Future Approaches to Changing Perceptions of Generic Drugs
Generic drugs save billions but still face distrust. Learn how new transparency tools, biosimilars, and patient education are changing perceptions - not by convincing people, but by letting them experience the truth.
FDA Bioequivalence Standards for NTI Drugs: What You Need to Know
The FDA applies stricter bioequivalence standards for narrow therapeutic index (NTI) drugs like warfarin, phenytoin, and digoxin to prevent dangerous dosing errors. Learn how these rules differ from regular generics and what they mean for patients.