Serious Adverse Event Quiz
How Well Do You Understand Serious Adverse Events?
The FDA defines 'serious' based on specific outcomes, not just how severe symptoms feel. Test your knowledge with these scenarios:
Question 1
You develop severe nausea after starting a new medication that requires you to take a day off work but you don't go to the hospital.
Question 2
You experience a severe rash after taking a new drug that causes you to go to the emergency room for treatment.
Question 3
You have a mild headache after starting a new medication that resolves with an over-the-counter pain reliever.
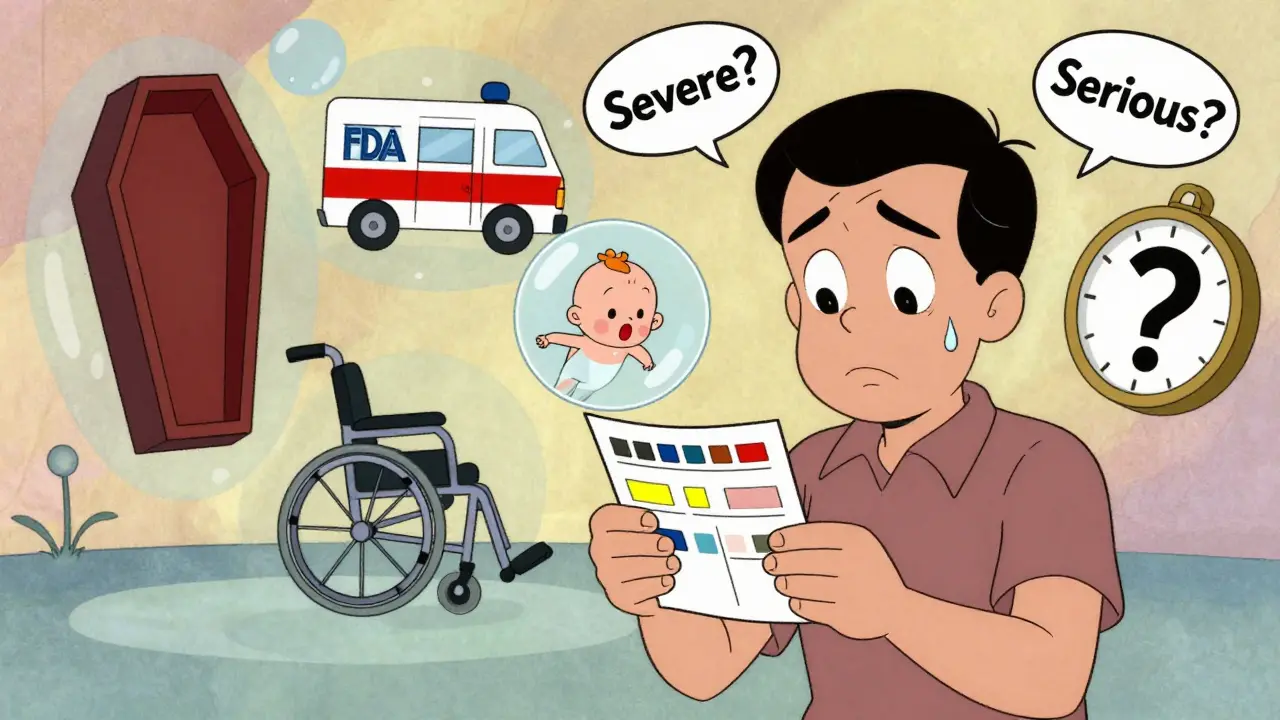
When you’re taking a new medication or joining a clinical trial, you might see the term serious adverse event in your consent forms or medication guides. It sounds scary. And if you’re like most patients, you probably think it means a bad side effect - maybe something really painful or intense. But here’s the truth: serious doesn’t always mean severe. And that difference could change how you understand your own health risks.
What Exactly Is a Serious Adverse Event?
The U.S. Food and Drug Administration (FDA) has a very specific definition for a serious adverse event (SAE). It’s not just any unpleasant reaction. An event is classified as serious only if it leads to one of five clear outcomes:- Death - even if it’s suspected, not confirmed, that the drug caused it
- Life-threatening - meaning you were in danger of dying at the time it happened
- Hospitalization - either you had to be admitted, or your stay was extended by at least 24 hours
- Permanent disability or damage - something that seriously disrupts your daily life, like losing the ability to walk or see clearly
- Birth defect - if you’re pregnant or planning to be, and the drug might have harmed the baby
There’s also something called an Important Medical Event - a situation that doesn’t fit neatly into those five categories but could become serious if not treated. For example, a sudden drop in blood pressure that doesn’t land you in the hospital but requires emergency treatment to prevent collapse. Those count too.
This system isn’t about how much pain you feel. It’s about what actually happened to your body or your life. A bad headache? Not serious. A headache that causes you to go to the ER because you can’t think straight? That could be.
Why This Matters More Than You Think
You might wonder why this matters if you’re just trying to decide whether to take a pill. Here’s the real impact: this classification drives what the FDA does next.In 2022 alone, the FDA used SAE reports to issue 128 safety alerts and change the warning labels on 47 medications. That means real changes to how drugs are prescribed - like adding new warnings about heart risks or removing a drug from the market entirely.
Take the case of a diabetes medication that caused rare but fatal liver damage. Early SAE reports from clinical trials flagged it. Without that system, those cases might have been dismissed as rare accidents. Instead, the FDA added a black box warning - the strongest kind - and doctors now test liver function before prescribing it.
But here’s the catch: this system only works if people report what happens. The FDA gets most of its data from doctors and drug companies. Patients? They rarely report. In fact, experts estimate that only 1% to 10% of all adverse events are ever reported. That means a lot of risks go unnoticed - especially for side effects that don’t land you in the hospital.
What’s the Difference Between Serious and Severe?
This is where most patients get confused.“Severe” describes how intense a side effect feels. The National Cancer Institute uses a five-level scale:
- Grade 1: Mild - annoying, but you can still do your normal activities
- Grade 2: Moderate - you need a little help, like an over-the-counter pill or a quick doctor visit
- Grade 3: Severe - you need medical help, maybe even hospitalization
- Grade 4: Life-threatening - you’re in intensive care
- Grade 5: Fatal
Now here’s the twist: a Grade 3 side effect - say, severe nausea that makes you miss work - is not automatically a serious adverse event. If it doesn’t require hospitalization or cause permanent damage, it’s not classified as serious by the FDA. But it’s still very real. You felt awful. You couldn’t function.
Conversely, a Grade 1 side effect - like a mild rash - could be serious if it leads to a hospital stay because you had an allergic reaction. That’s why the FDA doesn’t rely on how bad you feel. It relies on what actually happened to your health.
A 2022 survey of 1,543 patients who’d been in clinical trials found that 78% mixed up “serious” and “severe.” Many panicked when they saw “Grade 3 fatigue” listed as an adverse event, thinking it meant they were in danger. Their nurse had to explain: “It’s not serious - it’s just really tiring.”

What You Should Look For in Your Medication Guide
When you get a new prescription, you’ll get a medication guide. It’s usually a small paper insert. Don’t skip it. Look for a section called “Warnings and Precautions.” That’s where the FDA requires drugmakers to list the serious adverse events seen during testing.For example, a cancer drug might say: “Serious infections occurred in 2.3% of patients.” That doesn’t mean you’ll get sick. It means, out of every 100 people who took the drug, about 2 had infections so bad they needed hospital care or IV antibiotics.
In clinical trial consent forms, look for the “Risks and Discomforts” section. It should explain how side effects will be tracked and what counts as serious. If it doesn’t, ask. You have the right to understand what you’re signing up for.
The FDA now recommends that drug companies use plain language in these documents. So instead of “SAE,” they should say: “An event that results in death, hospitalization, or permanent damage.” That’s much clearer.
How You Can Help Improve Drug Safety
You don’t have to wait for your doctor to report a side effect. You can report it yourself.The FDA’s MedWatch program lets patients report adverse events directly using Form 3500B. You can do it online, by mail, or by phone. In 2022, the FDA received 38,452 reports from patients - up 12% from the year before.
What should you report? Anything that fits the five criteria above. Even if you’re not sure. If you had to go to the ER after starting a new pill, report it. If you had a reaction that made you miss work for a week, report it. If your child developed a rash that turned into a hospital visit, report it.
These reports matter. They help the FDA spot patterns. One patient’s story might be the clue that leads to a life-saving change.

What’s Changing Soon
The FDA knows patients don’t understand this system well. That’s why they’re making changes.In 2023, they released a draft plan to simplify how SAEs are described in patient materials. By 2025, all clinical trial websites will need to include plain-language summaries of serious side effects - no jargon.
They’re also using AI to sort through reports faster. Right now, it takes weeks for the FDA to review every report. With new tools, critical events can be flagged in days. And by the end of 2024, they plan to launch a free patient education portal - with videos, checklists, and real stories - to help you understand what serious means.
One of the biggest shifts is listening to patients. In the past, the FDA decided what was serious based on clinical data. Now, they’re asking patients: “What would scare you? What would make you stop taking a drug?” That’s changing how risks are weighed. For example, some patients say losing their hair is devastating - even if it’s not life-threatening. The FDA now considers that in their evaluations.
What to Do Next
If you’re on a new medication:- Read the medication guide. Look for the “Warnings” section.
- Ask your pharmacist: “What are the serious side effects I should watch for?”
- Know the difference: Severe = how bad it feels. Serious = what it does to your health.
- If something happens that fits the five criteria - report it to MedWatch.
- Keep a simple log: Date, symptom, how long it lasted, what you were taking.
You don’t need to be a scientist to help protect your health. You just need to know what to look for - and when to speak up.
Is a serious adverse event the same as a bad side effect?
No. A bad side effect might be uncomfortable, like nausea or headaches. A serious adverse event is defined by specific outcomes: death, hospitalization, life-threatening danger, permanent damage, or birth defects. Many side effects are unpleasant but not serious.
If I have a Grade 3 side effect, does that mean it’s serious?
Not necessarily. Grade 3 means it’s severe - you may need medical help or hospital care. But it only counts as a serious adverse event if it actually leads to hospitalization, permanent damage, or another of the five FDA-defined outcomes. For example, severe fatigue that doesn’t require hospitalization is Grade 3 but not serious under FDA rules.
Can I report a side effect myself?
Yes. The FDA’s MedWatch program lets patients report adverse events directly. You can file a report online at fda.gov/medwatch or by calling 1-800-FDA-1088. Even if you’re unsure whether it’s serious, report it. Your report could help identify a pattern others are missing.
Why do some side effects show up in my trial results but aren’t labeled serious?
Because the FDA only labels events as serious if they meet specific criteria - like hospitalization or death. Many side effects are severe (Grade 3 or 4) but not serious. For example, low blood counts in cancer patients are common and often treated without hospitalization. They’re listed as adverse events, but not as serious unless they lead to infection, bleeding, or other complications that meet the criteria.
What should I do if I think a medication caused a serious event?
First, contact your doctor immediately. Then, report it to the FDA through MedWatch. Don’t wait. Even if you’re not sure, your report adds to the data that helps the FDA spot risks. Also, keep a record: write down the date, what you were taking, what happened, and how long it lasted. That info helps doctors and regulators make better decisions.







kenneth pillet
Read the guide. Look at the warnings. Report anything that feels off. Simple. No need to overthink it. I’ve been on three clinical trials and this is how I stay safe.
Stacey Marsengill
They call it 'serious' but they don’t care until someone dies. I had a rash after my chemo drug-no hospital, no ER, just a burning nightmare that lasted three weeks. They didn’t count it. But I still wake up thinking about it. They’re not protecting us. They’re protecting the bottom line.
Tyler Myers
Let me break this down for you folks who think the FDA gives a damn. This whole 'serious adverse event' thing? It’s a legal loophole. Drug companies report what they have to, not what they should. The FDA? They’re underfunded and overworked. That 1% reporting rate? That’s not negligence-that’s the system being designed to fail. And now they want us to trust AI to fix it? Please. The same people who approved opioids are the ones running this show. You think they want to find more bad drugs? Or do they want to keep the cash flowing?
They added a black box warning to that diabetes drug? Only after three people died. Three. That’s the threshold. Not 'this made me vomit for a week.' Not 'this gave me panic attacks every night.' Only death. Or near-death. That’s the metric. And if you’re not dead yet? You’re just noise.
And don’t get me started on 'plain language.' They’ll say 'serious = hospitalization' but never mention that hospitalization means you had to pay $20k out of pocket. That’s the real cost. Not the label. Not the warning. The bill. That’s what makes it serious.
So yes, report your side effects. But don’t expect anything to change. You’re just feeding the machine. And it’s already full.
Praseetha Pn
Wait so if I get dizzy and fall down but don’t break anything or go to the hospital it’s not serious? That’s insane. My aunt took that blood pressure med and collapsed in the kitchen-no hospital, no ER, just her husband called 911 and they gave her fluids at home. She was out for 4 hours. That’s not ‘severe’? That’s not ‘serious’? What’s the point of even having a system if it ignores near-death experiences? They’re counting bodies, not near-misses. And that’s why people die later. Because no one saw the warning signs.
And why do they still use jargon like ‘SAE’ in consent forms? Who even says that? You’re handing a 72-year-old diabetic a 12-page document full of Latin and legal nonsense and expecting them to understand? That’s not informed consent. That’s informed consent theater.
I reported my own reaction after my antidepressant made me hallucinate for 3 days. Got a form letter back saying ‘thank you for your feedback.’ No follow-up. No call. Nothing. So I wrote to my senator. That’s when they finally responded. Not the FDA. My senator. That’s how broken this is.
rachel bellet
There is a critical misalignment between clinical grading scales (NCI CTCAE v5) and regulatory classification criteria (FDA 21 CFR 310.5). A Grade 3 event is operationally defined by objective clinical parameters requiring intervention, yet it does not automatically trigger SAE classification unless it meets the statutory thresholds for hospitalization, disability, or life threat. The conflation of subjective symptom severity with regulatory seriousness is a pervasive cognitive bias among lay populations, as evidenced by the 78% misclassification rate in the 2022 survey. This is not a communication failure-it is a systemic epistemological disconnect between clinical science and regulatory policy.
Furthermore, the reliance on passive patient reporting via MedWatch is statistically inadequate. The signal-to-noise ratio is so low that meaningful pharmacovigilance requires algorithmic triage, which is precisely why the FDA’s AI initiatives are not merely prudent-they are non-negotiable for public safety.
Naomi Keyes
Wait-I just looked at my new blood thinner’s guide, and it says ‘serious adverse events include bleeding requiring transfusion or hospitalization.’ But what if I bleed for 10 days and my doctor just gives me iron pills? No transfusion. No hospital. So it’s not serious? That’s not how my body works. I’m still weak. I’m still dizzy. I still can’t walk to the mailbox. But according to the FDA? I’m fine. That’s not safety. That’s paperwork.
And why do they say ‘birth defect’? What about miscarriage? What about preterm labor? Those aren’t listed. But they happen. And they’re devastating. So why isn’t that included? Are they only worried about babies born alive? What kind of logic is that?
Also-why does every single medication guide say ‘contact your doctor’? Who do they think we are? Doctors? We’re patients. We don’t know what to say. We don’t know what counts. We just know we feel awful. And we’re supposed to guess if it’s ‘serious’? That’s not informed consent. That’s a trap.
Danny Gray
Isn’t it funny how we’ve turned health into a bureaucratic game? We measure suffering by hospital beds and legal definitions instead of human experience. We say ‘serious’ when we mean ‘expensive to treat’ or ‘likely to trigger a lawsuit.’ But what about the quiet suffering? The insomnia. The anxiety. The loss of joy? The person who stops laughing because every pill makes them feel like a ghost? That’s not in the manual. But it’s real. And maybe the real failure isn’t in the reporting system-it’s in our refusal to see pain unless it fits a box.
Maybe ‘serious’ isn’t a medical term. Maybe it’s a moral one. And we’ve outsourced morality to a spreadsheet.
Chuck Dickson
You guys are right to be frustrated-but don’t give up. Reporting matters. I reported a weird rash after my new statin. Two months later, the FDA updated the warning. It wasn’t just me-hundreds of others did too. We’re not noise. We’re the early warning system. And yeah, the system’s broken. But if we don’t speak up, it stays broken. Start small: write down your symptoms. Take a photo of the rash. Save the pill bottle. Then go to MedWatch. It takes 10 minutes. You’re not just helping yourself-you’re helping someone’s mom, their brother, their kid. That’s power. Don’t underestimate it.
Aysha Siera
They know. They always know. They just wait. Wait for enough people to die. Then they act. Then they say they care. Then they put a sticker on the bottle. Then they go back to sleep. The system is not broken. It is working exactly as designed.