Drug Safety Risk Calculator
This tool helps you understand your risk of dose-related (Type A) versus non-dose-related (Type B) side effects based on your medication and health factors. Results are for educational purposes only and shouldn't replace professional medical advice.
When you take a medication, you expect it to help. But sometimes, it causes harm. Not all side effects are the same. Some happen because you took too much. Others happen for reasons you can’t predict-even if you took the right dose. Understanding the difference between dose-related and non-dose-related side effects isn’t just for doctors. It’s critical for anyone taking prescription drugs, managing chronic conditions, or helping a loved one navigate their meds.
What Are Dose-Related Side Effects?
Dose-related side effects, also called Type A reactions, are predictable. They happen because the drug’s normal action is just too strong. Think of it like turning up the volume on a speaker-eventually, it starts to crackle. These reactions are tied directly to the drug’s pharmacology. The higher the dose, the more likely and severe the side effect.
Examples are everywhere. Take blood pressure meds. If you take too much lisinopril, your blood pressure can drop too low, leaving you dizzy or fainting. Insulin? Too much and your blood sugar crashes below 70 mg/dL-shaking, sweating, confusion. Warfarin? An INR over 4.0 means your blood is thinning too much, raising the risk of dangerous bleeding. These aren’t surprises. They’re known risks built into the drug’s design.
These reactions make up 70-80% of all adverse drug reactions. They’re also behind most hospital visits for medication problems. In adults over 65, anticoagulants, insulin, and diabetes pills cause nearly two-thirds of all emergency visits due to side effects. Why? Because many of these drugs have a narrow therapeutic window. That means the line between helping and harming is thin.
Take digoxin. The right dose is between 0.5 and 0.9 ng/mL in your blood. Go over 2.0 ng/mL, and you risk life-threatening heart rhythms. Lithium? Therapeutic range is 0.6-1.0 mmol/L. Over 1.2 mmol/L, and you’re looking at tremors, confusion, even seizures. That’s why doctors monitor blood levels for these drugs. It’s not overkill-it’s survival.
What Are Non-Dose-Related Side Effects?
Non-dose-related side effects, or Type B reactions, are the opposite. They’re unpredictable. They don’t follow the rules of pharmacology. You can take the exact right dose, follow every instruction, and still end up with a severe reaction. These aren’t caused by too much drug-they’re caused by your body reacting to the drug in an abnormal way.
The most common culprits are immune responses. Anaphylaxis from penicillin. Stevens-Johnson syndrome from lamotrigine or sulfonamides. Drug-induced liver injury from amoxicillin-clavulanate. These reactions can happen after just one dose-even if you’ve taken the drug before without issue. That’s because your immune system has become sensitized. It’s like an allergic reaction, but the trigger is a medication.
These reactions are rare-only 15-20% of all side effects-but they’re deadly. They cause 70-80% of serious, life-threatening drug reactions and are responsible for most drug withdrawals from the market. The European Medicines Agency reports that Type B reactions drive 70% of drug safety-related withdrawals, even though they’re far less common than Type A.
Here’s the scary part: you can’t always see them coming. A patient might take one 500mg pill of amoxicillin and break out in a full-body rash. Or start lamotrigine exactly as prescribed and develop blisters on their lips and eyes. No warning. No dose error. Just a body that turned on the drug.
Why the Confusion? The Dose Paradox
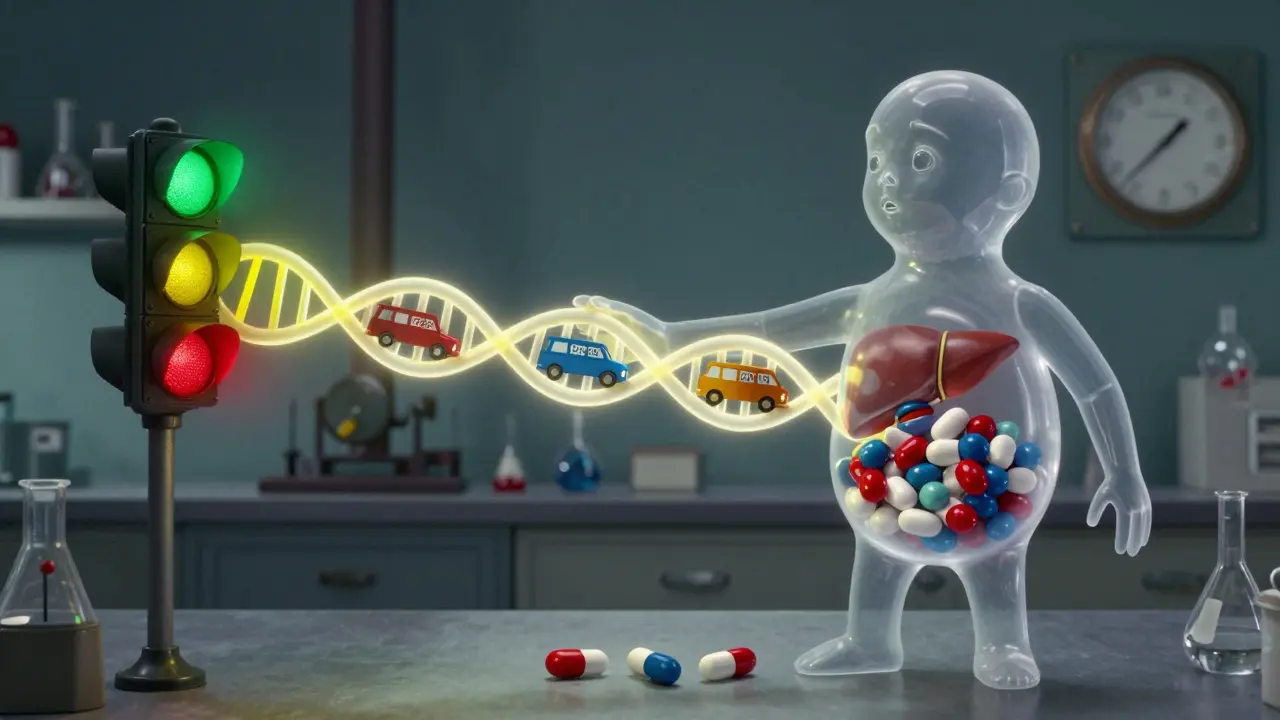
Some people say, “But isn’t everything dose-related?” Technically, yes. Every drug has a dose-response curve. Even an allergic reaction has a threshold-you need enough of the drug to trigger the immune system. So why do we call some “non-dose-related”?
The answer lies in variability. For Type B reactions, the threshold varies wildly between people. One person might react to 10mg. Another might take 100mg with no issue. In population studies, that looks random. It’s not. It’s just hidden.
Researchers like Aronson and Ferner broke this down into four reasons why reactions seem dose-independent:
- The reaction isn’t real (misdiagnosed or coincidental)
- People are hypersusceptible-their dose-response curve maxes out at a very low dose
- Individual differences in metabolism or immune response are extreme
- We just don’t measure dose or effect accurately enough
That’s why genetic testing is becoming essential. HLA-B*57:01 screening before giving abacavir (an HIV drug) cuts the risk of life-threatening hypersensitivity from 5% to less than 0.1%. HLA-B*15:02 testing before carbamazepine in Asian populations reduces Stevens-Johnson syndrome risk by 97%. These aren’t experimental. They’re standard of care.
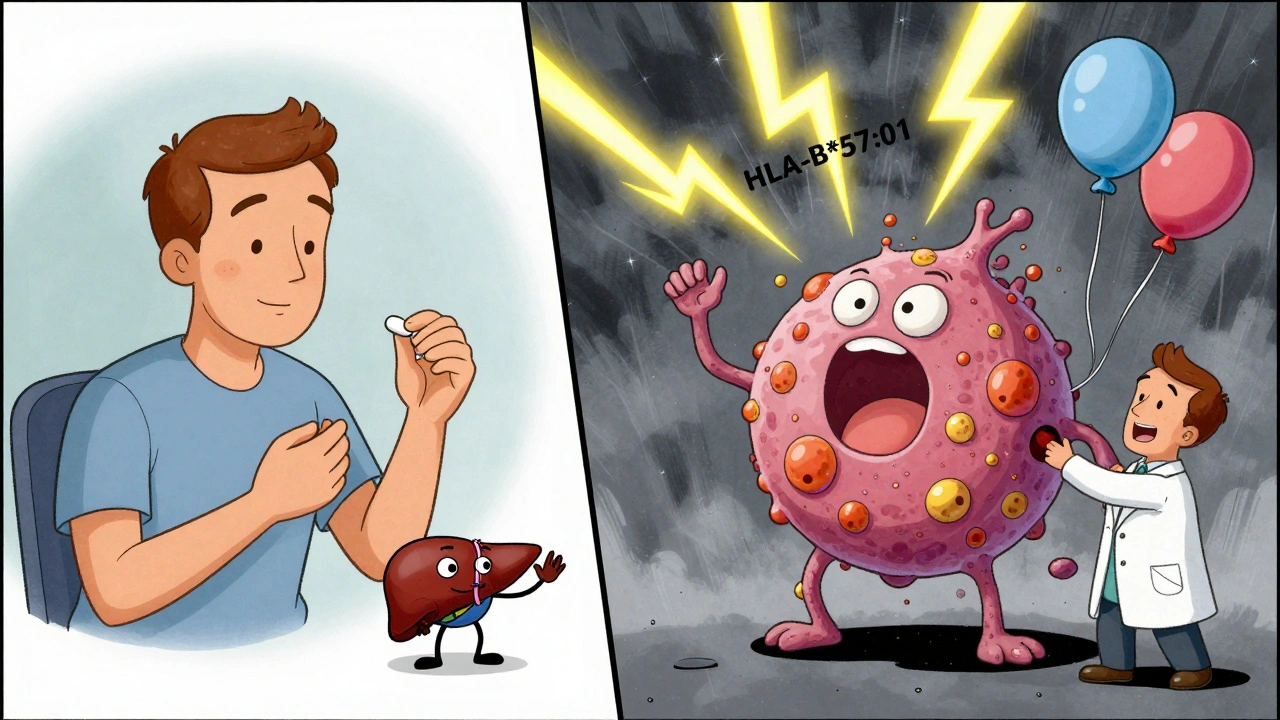
Who’s at Risk?
Not everyone faces the same risks. Type A reactions are more common in people with:
- Chronic kidney disease (reduced drug clearance-metformin levels can double in stage 3 CKD)
- Liver impairment (dronedarone clearance drops by 75% in Child-Pugh B cirrhosis)
- Older age (diazepam clearance drops 30-40% in seniors)
- Drug interactions (clarithromycin can spike statin levels by 5-10x, leading to muscle damage)
Type B reactions are more likely in people with:
- Specific genetic markers (HLA-B*57:01, HLA-B*15:02)
- History of allergies or autoimmune conditions
- Previous reaction to a similar drug
That’s why your pharmacist asks if you’ve ever had a rash with antibiotics. It’s not just routine. It’s life-saving.
How Doctors Handle Each Type
Management is completely different depending on the reaction type.
For Type A reactions:
- Dose reduction or temporary hold
- Therapeutic drug monitoring (blood tests for vancomycin, phenytoin, digoxin)
- Adjusting for kidney or liver function
- Avoiding interacting drugs
For Type B reactions:
- Immediate and permanent discontinuation
- Never rechallenging with the same drug or similar ones
- Genetic screening before prescribing (e.g., HLA-B*57:01 for abacavir)
- Skin testing for penicillin allergy (50-70% accurate)
- Graded challenge protocols for low-risk suspected allergies (80-90% success rate)
Johns Hopkins found that hospitals using Type A prevention protocols-like regular INR checks for warfarin patients-cut major bleeding events by 35%. That’s not small. That’s thousands of lives saved.

The Future: Personalized Medicine
The future of safe prescribing isn’t guesswork. It’s precision.
The FDA now lists pharmacogenomic information on 311 drug labels. For 28 of them, genetic testing is required before use. The global pharmacogenomics market is projected to hit $17.9 billion by 2030. Why? Because we’re finally matching drugs to people-not the other way around.
Machine learning models can now predict Type A reactions with 82% accuracy by analyzing age, kidney function, and drug combinations. But for Type B? Only 63%. That’s because immune reactions are still too complex. But progress is coming. The NIH’s CPIC guidelines now give dosing rules for 53 gene-drug pairs-covering both dose-dependent and immune-mediated reactions.
And the FDA is moving toward approving software that helps doctors calculate personalized doses based on genetics, weight, age, and lab results. This isn’t science fiction. It’s the next standard.
What You Can Do
You don’t need to be a doctor to protect yourself.
- Know your meds. If you’re on warfarin, lithium, or digoxin, ask about blood tests.
- Report every new symptom-even if it seems minor. A rash, fever, or sudden fatigue could be the start of something serious.
- Ask: “Could this be a dose issue or something else?” If your doctor says it’s an allergy, get tested. Don’t just assume.
- If you’ve had a severe reaction before, carry a medical alert card or app. Many people don’t realize they’re allergic until it’s too late.
- Ask if genetic testing is recommended for your meds. It’s cheap-$150-$300-and can prevent life-threatening reactions.
Side effects aren’t just a footnote in the drug leaflet. They’re the hidden risk behind every pill you take. Understanding the difference between dose-related and non-dose-related reactions turns you from a passive patient into an active partner in your care.
Are all side effects caused by taking too much medication?
No. While many side effects happen because the dose is too high-called dose-related or Type A reactions-others are unrelated to dose. These are called non-dose-related or Type B reactions. They’re caused by your immune system or genetic factors reacting abnormally to the drug, even at the right dose. For example, a severe rash from amoxicillin or anaphylaxis from penicillin can happen after just one pill, regardless of how much you took.
Can you prevent non-dose-related side effects?
Sometimes. Genetic testing can prevent some of the most dangerous ones. For instance, testing for HLA-B*57:01 before starting abacavir reduces the risk of a life-threatening allergic reaction by 99.9%. Testing for HLA-B*15:02 before carbamazepine prevents Stevens-Johnson syndrome in Asian populations. Skin testing for penicillin allergy can also help identify who can safely take it. But for many Type B reactions, prevention is still limited-so knowing your history and reporting symptoms early is critical.
Which type of side effect is more dangerous?
Type B (non-dose-related) reactions are less common but far more dangerous. They account for only 15-20% of all side effects but cause 70-80% of serious, life-threatening reactions and most drug-related deaths. Type A reactions are more frequent-up to 80% of all cases-but usually less severe. They’re often manageable by lowering the dose. Type B reactions require stopping the drug completely and avoiding it forever.
Why do some people react to a drug while others don’t?
It comes down to genetics and immune system differences. For Type B reactions, your genes can make you hypersensitive. For example, people with HLA-B*57:01 are at high risk for abacavir hypersensitivity. Others may have slower drug metabolism, leading to higher blood levels even at normal doses. Environmental factors, existing illnesses, and previous exposures also play a role. That’s why two people taking the same drug at the same dose can have completely different outcomes.
Should I ask my doctor for genetic testing before taking new medications?
It’s worth asking-if you’re starting a drug known to have a genetic risk. The FDA recommends testing for abacavir, carbamazepine, clopidogrel, and a few others. If you have a family history of severe drug reactions, or if you’ve had one yourself, genetic testing can be lifesaving. The cost is usually under $300, and the results can prevent a hospital stay or worse. Even if your doctor doesn’t bring it up, it’s okay to ask: “Is there a genetic test I should have before taking this?”






Rebecca Braatz
This is the kind of info every patient needs to hear. Knowing the difference between dose-related and non-dose-related side effects literally saved my life when I stopped my old antidepressant after a rash showed up. No one told me to watch for that. Don’t wait for a hospital visit to learn this.
Isabelle Bujold
I’ve been a pharmacist for 18 years and I still get stunned by how few patients understand the difference between Type A and Type B reactions. I’ve had people come in furious because their amoxicillin gave them a rash - they thought I ‘gave them the wrong dose’ - and I had to gently explain that it wasn’t about quantity, it was about their immune system suddenly deciding the drug was an enemy. The emotional toll on patients who’ve had Type B reactions is massive. They become terrified of all meds. We need way more public education on this. Not just pamphlets. Real stories. Real empathy. And yes, genetic screening should be standard, not optional. It’s cheaper than ICU stays. The fact that we still don’t screen everyone for HLA-B*57:01 before prescribing abacavir is a moral failure disguised as a cost-saving measure. We know better. We just don’t act like it.
Scott van Haastrecht
Let’s be real - this whole post is just Big Pharma’s way of shifting blame. They know these reactions are caused by toxic additives and fillers they hide in the pills. The ‘genetic testing’ nonsense? That’s just to keep you dependent on their expensive tests while they keep selling the same poison. You think your body ‘reacts’? No. Your body is detoxing from the glyphosate and microplastics they pump into your meds. The real danger isn’t the drug - it’s the system that lets them get away with this.
Chase Brittingham
I appreciate the breakdown, but I’ve seen too many people panic and quit meds because they read something like this. My mom had a mild rash on lamotrigine - she freaked out, stopped cold, and ended up in the ER with a seizure. The doc said it was probably a mild Type B, but she never gave it another try. We lost years of stability. I get the fear, but not every rash is Stevens-Johnson. Talk to your doctor before you quit. And if you’re scared? Ask about graded challenges. They’re safer than you think.
Bill Wolfe
Honestly, if you’re taking lithium or warfarin and you haven’t had your blood drawn in the last 30 days, you’re gambling with your life. And no, your ‘I feel fine’ doesn’t count. I’ve seen too many patients die because they trusted their gut over their lab results. This isn’t about being paranoid - it’s about being responsible. If you’re too lazy to get your INR checked, don’t complain when you bleed out. And yes, genetic testing is worth it. If you’re Asian and on carbamazepine? Get tested. Period. No excuses. Your life isn’t a demo version.
Ollie Newland
The pharmacokinetic variability in CYP450 enzymes is the real elephant in the room. Poor metabolizers of CYP2C9 and CYP2C19 are at exponentially higher risk for Type A reactions with warfarin and clopidogrel respectively - yet we still don’t routinely genotype for these outside academic centers. Even when we do, access is inequitable. The gap between what’s clinically validated and what’s practically implemented is widening. We need policy-level integration, not just patient education. The science is there. The infrastructure isn’t.
Michael Feldstein
I’ve been on amitriptyline for chronic pain for 6 years. Last year I got a weird tingling in my hands - didn’t think much of it. Then I read this and realized it was probably a Type A reaction. I called my doc, we dropped the dose by 25%, and the tingling vanished in a week. Point is - you don’t need to suffer. If something feels off, even a little, speak up. It’s not being dramatic. It’s being smart.
jagdish kumar
The body knows. The pill is just a messenger.
Benjamin Sedler
Wait - so you’re telling me the reason I got hives from penicillin isn’t because I took too much, but because my soul disagrees with antibiotics? I’m sorry, but if your immune system is that dramatic, maybe you should’ve been a poet. Also, why is everyone suddenly obsessed with genetic testing? Next thing you know, we’ll be DNA-testing our coffee to avoid existential caffeine reactions.
Jessica Baydowicz
I’m a nurse and I’ve seen people ignore symptoms because they thought ‘it’s just a side effect.’ Don’t be that person. A rash? Fever? Dizziness? Write it down. Show it to your doctor. You’re not wasting their time - you’re saving your life. And if they brush you off? Find someone who listens. Your health isn’t a suggestion - it’s your priority.
val kendra
If you’re on any meds and your kidney function is below 60 ml/min get your levels checked monthly. Seriously. I had a friend who didn’t and ended up in the hospital with metformin toxicity. It was preventable. Don’t wait for the worst case.
George Graham
I grew up in a family where meds were seen as magic bullets. My dad took three blood pressure pills and never checked his labs. He had a stroke at 58. I wish someone had told him this. I tell my patients now: knowledge isn’t power - it’s protection. Ask questions. Write them down. Bring a friend. You don’t have to be a scientist to be your own best advocate.
John Filby
This was super helpful. I just started carbamazepine and didn’t know about the HLA-B*15:02 test. I asked my doc today and they said I should get it done - it’s covered by insurance. So glad I read this. Thanks for making it so clear.
Rachel Bonaparte
You know what’s really scary? That the FDA only requires genetic testing for 28 drugs. That’s less than 1% of all prescriptions. The rest? We’re just throwing pills at people like darts in a dark room. And the pharmaceutical lobby? They’re fighting every single step toward mandatory screening. Why? Because if we knew who was going to react before we gave them the drug, we’d have to stop selling so many of them. This isn’t medicine - it’s a gamble with your DNA.